-
Medical Treatment Guidance
-
Medical Course
- Gastroenterology
- Pulmonology
- Nephrology
- Cardiology
- Endocrinology
- Internal Physical Examination
- Surgery
- Obstetric & Gynecology
- Pediatrics
- Neurosurgery
- Neurology
- Orthopedics
- Emergentology
- Laboratory Medicine
- Dentistry
- Radiology
- Urology
- Anesthesiology
- Occupational and Environmental Medicine
- Geratology
- Otolaryngology
- International Medical Center
- Medical Team
- Clinic Hours
-
Medical Course
-
Specialized Centers
- Specialized Centers
- 소화기 Endoscopy Center
- hemodialysis Center
- Laparoscope 수술 Center
- Breast·Thyroid Center
- Spinal 통증 Center
- Joint Center
- 뇌 Center
- 수술 Center
- 집중치료 Center
- Cardiovascular Center
- 내분비 Center
- Growth-Clinic Center
- 건강증진 Center
- Home Nursing Center
- Emergency Medical Center
- 진료협력 Center
- Clinical Trial Center
- Occupational Safety and Health Center
- 소화기 Endoscopy Center
- hemodialysis Center
- Laparoscope 수술 Center
- Breast·Thyroid Center
- Spinal 통증 Center
- Joint Center
- 뇌 Center
- 수술 Center
- 집중치료 Center
- Cardiovascular Center
- 내분비 Center
- Growth-Clinic Center
- 건강증진 Center
- Home Nursing Center
- Emergency Medical Center
- 진료협력 Center
- Clinical Trial Center
- Occupational Safety and Health Center
- Specialized Centers
- Customer Center
- About Clinic
- 홈페이지이용안내
- 회원 서비스
- 마이페이지
-
Medical Treatment Guidance
-
Medical Course
- Gastroenterology
- Pulmonology
- Nephrology
- Cardiology
- Endocrinology
- Internal Physical Examination
- Surgery
- Obstetric & Gynecology
- Pediatrics
- Neurosurgery
- Neurology
- Orthopedics
- Emergentology
- Laboratory Medicine
- Dentistry
- Radiology
- Urology
- Anesthesiology
- Occupational and Environmental Medicine
- Geratology
- Otolaryngology
- International Medical Center
- Medical Team
- Clinic Hours
-
Medical Course
-
Specialized Centers
- Specialized Centers
- 소화기 Endoscopy Center
- hemodialysis Center
- Laparoscope 수술 Center
- Breast·Thyroid Center
- Spinal 통증 Center
- Joint Center
- 뇌 Center
- 수술 Center
- 집중치료 Center
- Cardiovascular Center
- 내분비 Center
- Growth-Clinic Center
- 건강증진 Center
- Home Nursing Center
- Emergency Medical Center
- 진료협력 Center
- Clinical Trial Center
- Occupational Safety and Health Center
- 소화기 Endoscopy Center
- hemodialysis Center
- Laparoscope 수술 Center
- Breast·Thyroid Center
- Spinal 통증 Center
- Joint Center
- 뇌 Center
- 수술 Center
- 집중치료 Center
- Cardiovascular Center
- 내분비 Center
- Growth-Clinic Center
- 건강증진 Center
- Home Nursing Center
- Emergency Medical Center
- 진료협력 Center
- Clinical Trial Center
- Occupational Safety and Health Center
- Specialized Centers
- Customer Center
- About Clinic
- 홈페이지이용안내
- 회원 서비스
- 마이페이지
-
Medical Treatment Guidance
-
Medical Course
- Gastroenterology
- Pulmonology
- Nephrology
- Cardiology
- Endocrinology
- Internal Physical Examination
- Surgery
- Obstetric & Gynecology
- Pediatrics
- Neurosurgery
- Neurology
- Orthopedics
- Emergentology
- Laboratory Medicine
- Dentistry
- Radiology
- Urology
- Anesthesiology
- Occupational and Environmental Medicine
- Geratology
- Otolaryngology
- International Medical Center
- Medical Team
- Clinic Hours
-
Medical Course
-
Specialized Centers
- Specialized Centers
- 소화기 Endoscopy Center
- hemodialysis Center
- Laparoscope 수술 Center
- Breast·Thyroid Center
- Spinal 통증 Center
- Joint Center
- 뇌 Center
- 수술 Center
- 집중치료 Center
- Cardiovascular Center
- 내분비 Center
- Growth-Clinic Center
- 건강증진 Center
- Home Nursing Center
- Emergency Medical Center
- 진료협력 Center
- Clinical Trial Center
- Occupational Safety and Health Center
- 소화기 Endoscopy Center
- hemodialysis Center
- Laparoscope 수술 Center
- Breast·Thyroid Center
- Spinal 통증 Center
- Joint Center
- 뇌 Center
- 수술 Center
- 집중치료 Center
- Cardiovascular Center
- 내분비 Center
- Growth-Clinic Center
- 건강증진 Center
- Home Nursing Center
- Emergency Medical Center
- 진료협력 Center
- Clinical Trial Center
- Occupational Safety and Health Center
- Specialized Centers
- Customer Center
- About Clinic
- 홈페이지이용안내
- 회원 서비스
- 마이페이지
IRB
According to the related laws in order to protect the participant’s rights, safety, welfare, we installed the Institutional Review Board(a.k.a IRB) that is independently operated under Medical corporation Seok Gyeong Medical Foundation Central Hospital Clinical Trial Center’s chairman(head of organization or chief director). Our hospital try to proceed every human subjected and human-derived researches proper to the international standards of GCP(International Conference on Harmonisation - Good Clinical Practice, ICH GCP). We aim to contribute in protection and welfare promotion of the rights, dignity, safety, peace in every clinical trial or human subjected research participants. For a trial that observes of instructions in research ethics, we provide researchers about necessary informations and make them get prior approval from the IRB.
CENTRAL
Institutional Review Board
Missions of IRB
Under the International Conference on Harmonisation(ICH), Korea Good Clinical Practice(KGCP), laws related to bioethics and safety based on the Declaration of Helsinki, we plan to promote every participant’s rights, safety, welfare who are participating in human subjected, human-derived researches and increase the reliability of the research.
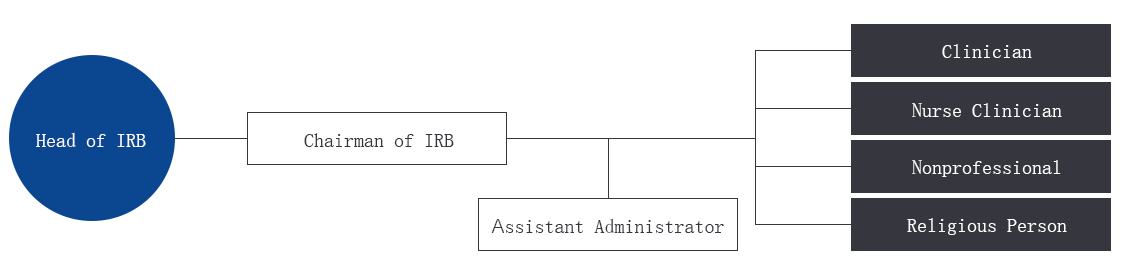
IRB Organization chart
행정간사 : +82 31-8031-3908

 한국어
한국어 English
English 中文
中文